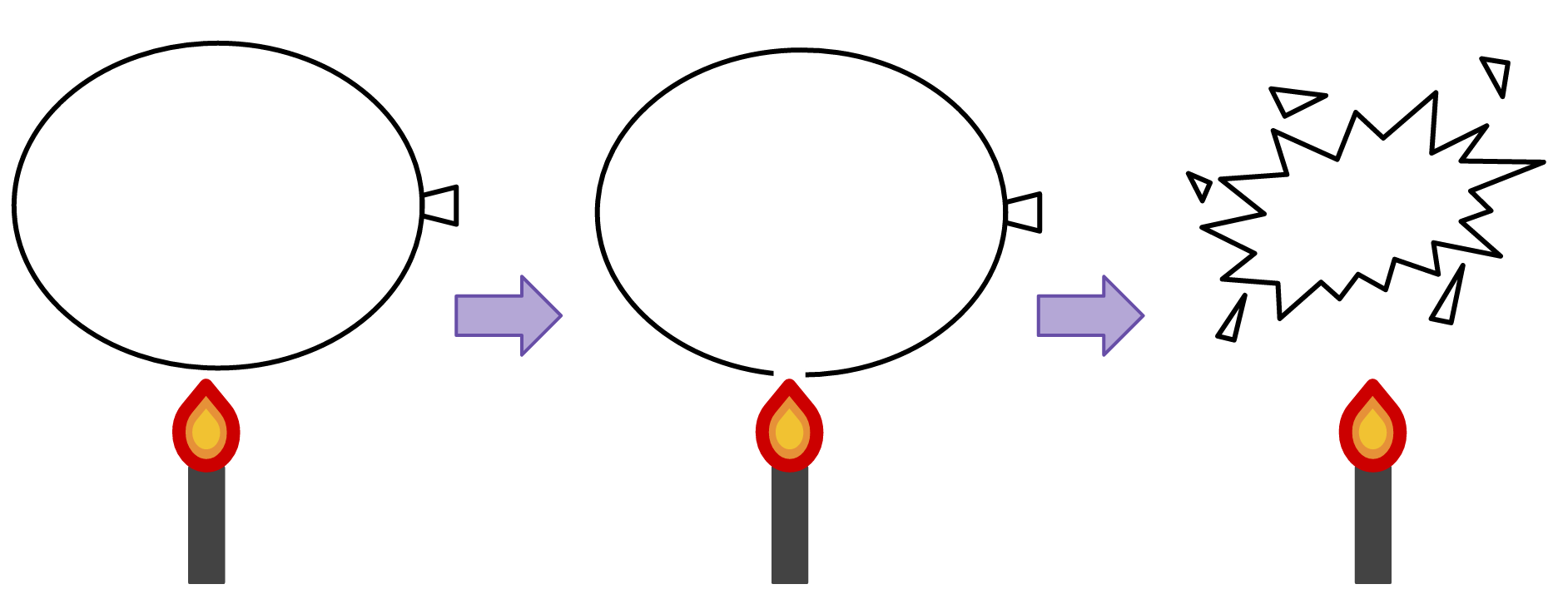
A balloon will pop when heated, but a balloon with some water in it will not.
Watch The Video:
Teachable Topics:
- convection
- heat transfer
- specific heat
Theory:
A balloon filled with air will pop when exposed to flame. The heat from the fire melts the latex, breaching the skin of the balloon.
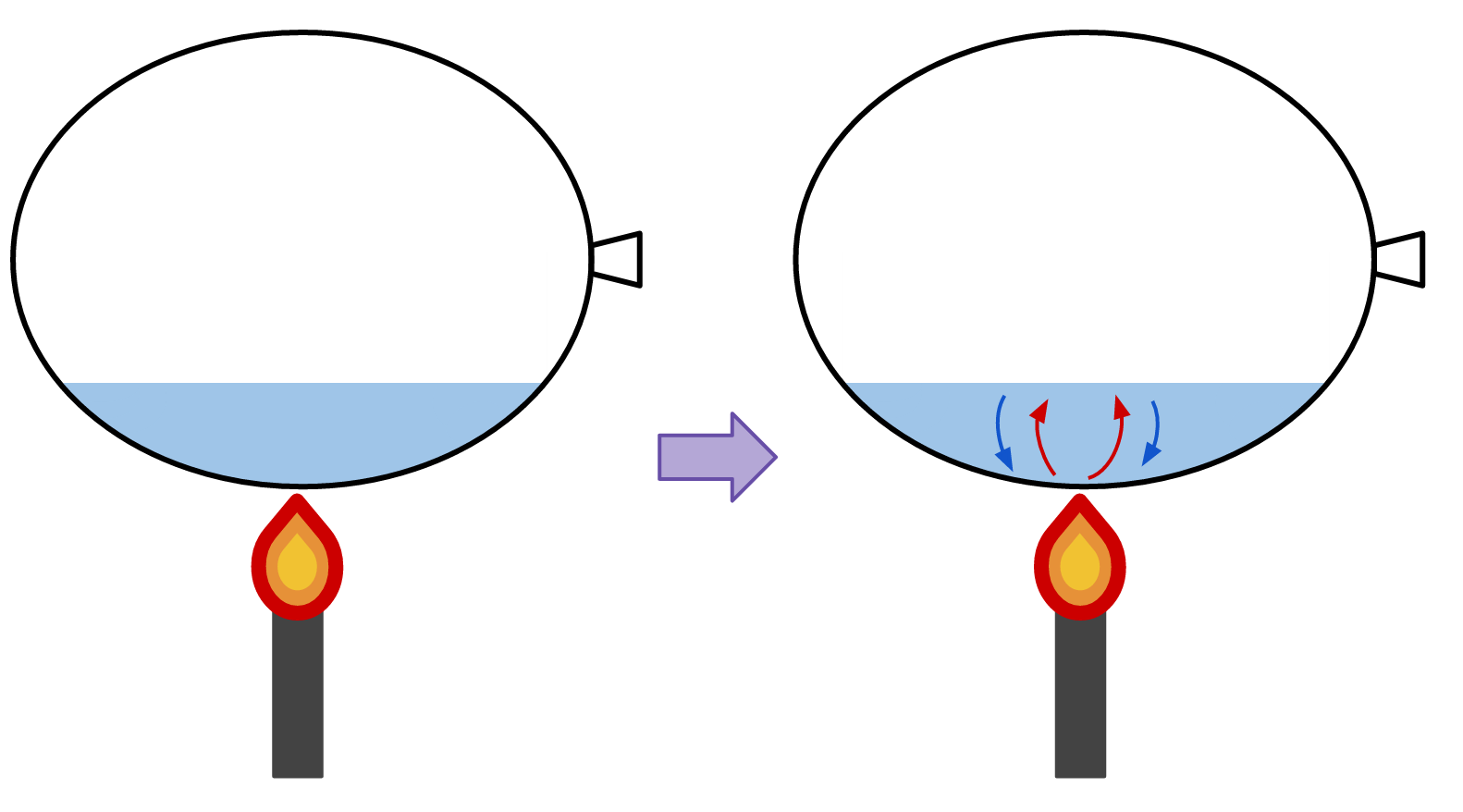
However, if the balloon is filled with some water, it will not pop when exposed to flame.
The heat from the flame passes through the balloon and into the water, so the balloon skin does not absorb the energy and does not melt/burst.When the water absorbs the energy, the water inside the balloon convects. Cool water at the bottom of the balloon absorbs heat and rises. It is then replaced by cool water which rushes to the bottom of the balloon. This cool water then absorbs heat and rises, to be replaced by more cool water, and so on... The convective property of water allows it to be cycled such that cool water is always closest to the heat source, and the warm water moves away. Water is perpetually carrying energy away from the balloon skin so the balloon does not absorb it and pop.
Apparatus:
- balloon
- water
- candle
Procedure:
- blow up a balloon and pour some water in it before tying it off
- hold the water-laden balloon over a flame and watch as it doesn't pop!