See the work done by an expanding volume.
Watch The Video:
Teachable Topics:
- work
- pressure
- atomic motion
Theory:
When gases cool they contract, and as they warm up they expand. This property allows gases to perform work simply by undergoing a temperature change.
When the balloon is immersed in liquid nitrogen, the air inside the balloon is cooled. This causes it to contract, and the balloon shrivels up.
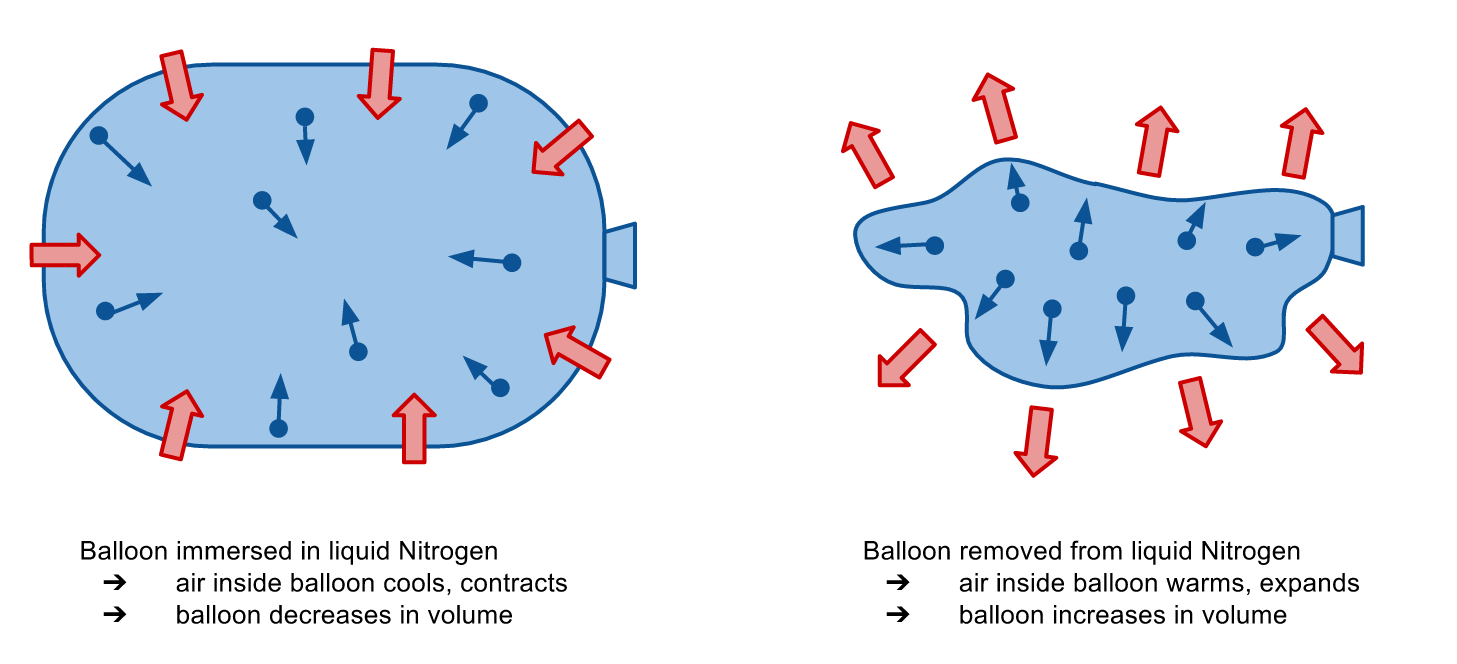
When the balloon is removed from the liquid nitrogen and placed on the platform, the cold air in the balloon comes to equilibrium with the warmer air in the room. The air in the balloon warms up and expands, restoring the balloon to its former glory. In the process, the masses atop the balloon are raised higher, increasing the potential energy of the system. This constitutes work, which is done by the expansion of the air in the balloon.
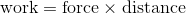
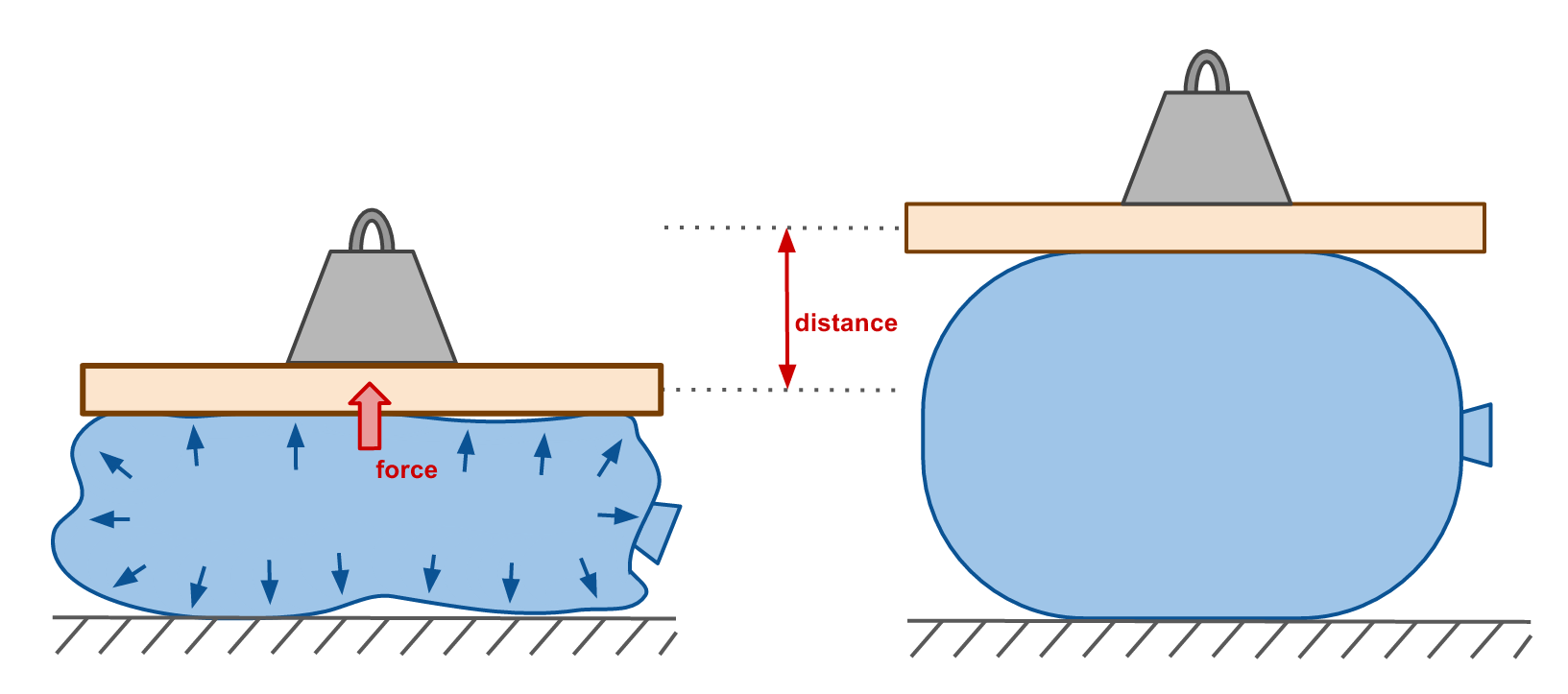
This is the same mechanism that that powers heat engines: air is compressed and expands to convert thermal energy into mechanical work.
Apparatus:
- balloon
- liquid nitrogen
- safety goggles
- cryogenic gloves
- a platform with masses
Procedure:
- inflate a ballon and cover in liquid nitrogen to cool the air inside
- this causes the balloon to contract as the cold air compresses
- remove the balloon from the liquid nitrogen and place under platform bearing masses
- as the air inside the balloon warms up to room temperature the balloon expands, raising the masses on the platform
Safety:
- Be very careful when using liquid nitrogen: wear safety goggles and cryogenic gloves.