As the gas in a pop can suddenly condenses, the pressure drops enough to crush it!
WATCH THE VIDEO!
And here it is again in better quality slo-mo...
Teachable Topics:
- gas expansion
- temperature and pressure
Theory:
An open can contains air at the same temperature and pressure as the atmosphere. That is, air molecules collide with the inside and the outside walls of the can and exert equal amounts of pressure there.
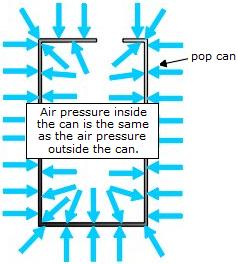
When the can is heated, the water in the bottom boils and turns into H2O gas (i.e. water vapour). A hole in the top of the can keeps the pressure balanced with the atmospheric pressure.
The balance can be taken away by immersing the can in a pool of ice water. The vapour inside cools and turns back into a liquid again. With less gas inside the can, the pressure on the inside can wall drops suddenly, and the atmosphere crushes the can.
Apparatus:
- one soda can
- a large tray filled with cold water
- a hot plate
- oven mitts

Procedure:
- Pour a little water into an empty soda can. You don't even need enough to cover the entire bottom.
- Heat the can on the hot plate and allow the water to come to a boil.
- While wearing the oven mitts, pick up the pop can and submerge the end with the hole into the cold water, and the atmosphere will crush the can.
Tips:
- Ice in the water will help keep it cold.
- Heat several cans at the same time so you can repeat the demonstration several times.
- Try crushing a can that doesn't have water in it. Theory tells us the hot air should contract when it is cooled, but it doesn't contract enough to cause the can to be crushed. You need water vapour condensing if you want to change the pressure enough to do that.



