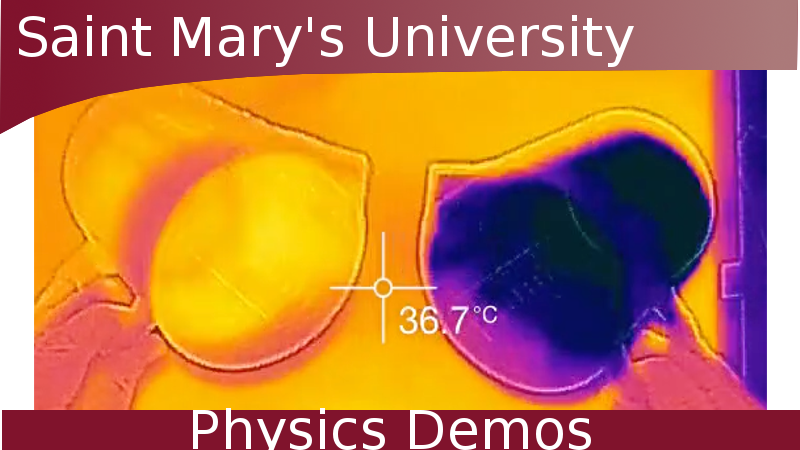
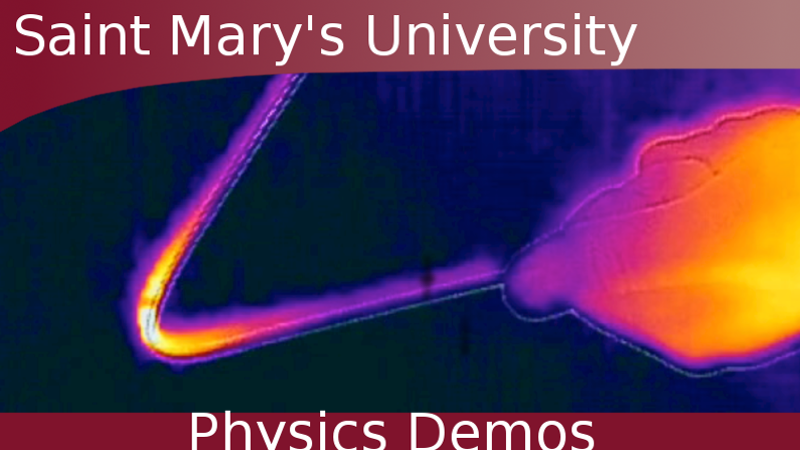
Collisions can create enough energy to burn a hole in paper.
Watch The Video:
Teachable Topics:
- heat transfer
- conservation of energy
- infrared vision
Theory:
In a closed system energy will be conserved; energy cannot be created or destroyed, it may only be converted from one form to another. This experiment demonstrates an example of this, converting kinetic energy into thermal energy. Kinetic energy is energy that an object possesses due to its motion. It is expressed as:
Ek = 1/2 m v2
where m is the mass of the object and v is its velocity. Thermal energy is energy a system posses due to its temperature.
The first law of thermodynamics, a version of the conservation of energy law, states:
dU = δQ - δW
where dU is the change in internal energy of a system, δQ is the energy added to the system, and δW is the energy lost by the system to the surroundings.
Apparatus:
- two chromium steel balls
- infrared camera
- printer paper
Procedure:
- Position a piece of printer paper vertically and point the infrared camera at it
- Crash the two steel balls together on either side of the paper
- The collision, if done with sufficient energy, will burn a hole in the paper and a small heat bloom will be visible in the infrared