What causes water to bead up on some surfaces and not on others?
Watch The Video:
 |
 |
 |
|
Bernoulli's Ping-Pong Ball |
Dancing Water Droplets |
Water as a Fibre-Optic |
Teachable Topics:
- chemical bonds
- polar molecules
- dipoles
Theory:
Hydrophobicity is the repulsion between water and another molecule (a hydrophobe).
Water is a polar molecule, meaning that is has a net dipole due to its configuration. A net dipole means that one side of the molecule is positively charged and the other side is negatively charged. The atoms in a polar molecule are arranged such that they do not cancel one another out, so each side of the molecule is oppositely charged.
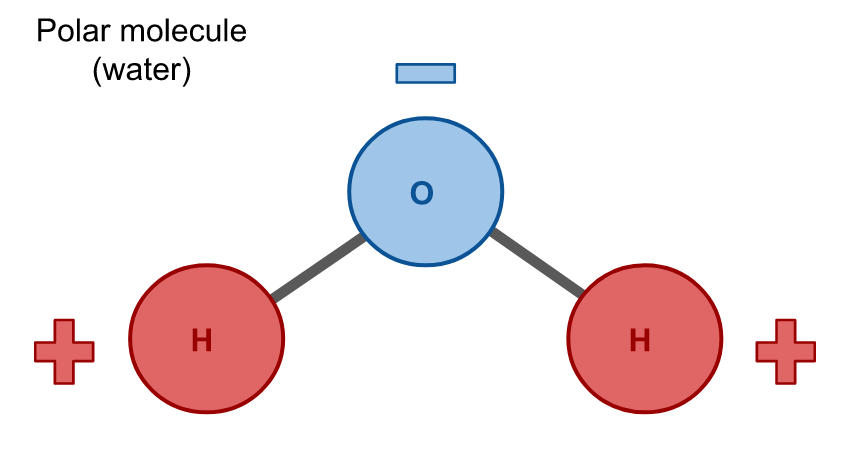
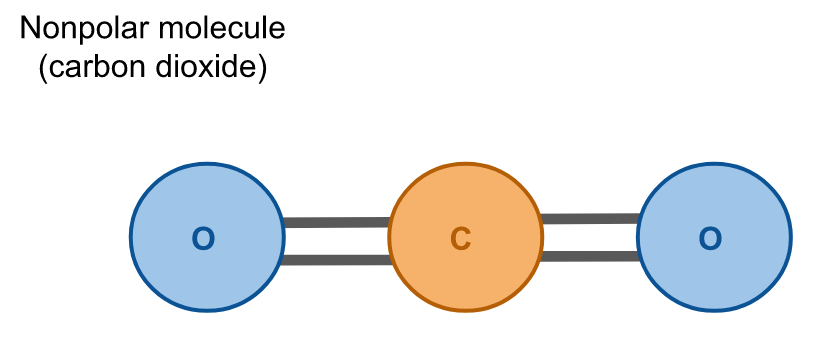
Hydrophobes are nonpolar molecules, which means that their atoms are arranged symmetrically such that there is no net dipole; all charges in the molecule cancel due to their geometry. Carbon dioxide (CO2) is an example of a nonpolar molecule. Like water, it is composed of three atoms, but unlike water these atoms have a linear configuration so their charges cancel and there is no net dipole.
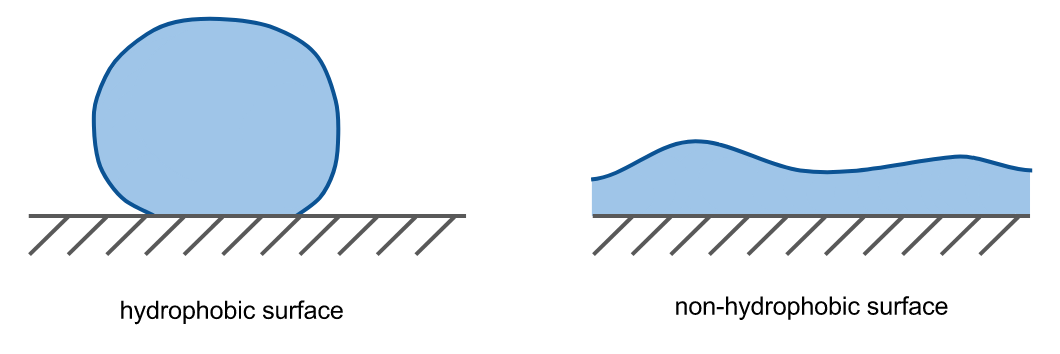
Hydrophobicity is the repelling that occurs between water and nonpolar molecules. When water is in contact with a hydrophobic material the bonds between the water molecules maximize, minimizing the surface area of the water that is in contact with the hydrophobic material.
Because water molecules exhibit a dipole (have a negative charge on one side and a positive charge on the other side), the attraction between water molecules is stronger than the attraction between a water molecule and a nonpolar molecule.
Nonpolar molecules prefer nonpolar molecules, while polar molecules prefer polar molecules. There is not a repulsive force between polar and nonpolar molecules, simply stronger bonds between the same types of molecules.
This causes nonpolar substances to cluster together when mixed with water (e.g. oil in water), and water to “bead up” atop a nonpolar surface.
Apparatus:
- mineral oil
- water
- water dropper
- glass sheet
Procedure:
- coat half the glass sheet in a layer of mineral oil, leaving the other half bare
- using the dropper, place a few drops of water on each half of the sheet
- watch the different behaviour of water on each surface



